Laboratories Receive ISO 15189 Accreditation in Malawi for the First Time

Four Malawi laboratories supported by the University of Maryland, Baltimore’s (UMB) Center for International Health, Education, and Biosecurity (Ciheb) have received accreditation from the Southern African Development Community Accreditation Service (SADCAS), becoming the first laboratories in the country to attain this status. ISO 15189 is an international standard specific to medical laboratories.
"I am very excited about this achievement, as we have come a long way. The achievement has shown that the laboratory quality standards in the country are internationally accepted, and the quality of results being generated by our laboratories are meeting the specific requirements, hence our contribution to the delivery service in the country is going well."
—Acting Deputy Director of MOH Diagnostics Department Joseph Bitilinyu
The Centers for Disease Control and Prevention and the Malawi Ministry of Health (MOH) began the process of lab accreditation more than five years ago and brought UMB on as an implementing partner in 2019. UMB, through Ciheb’s AMPLIFY project, provides technical assistance at the national and county level to strengthen the capacity of molecular laboratories in Malawi and increase access to quality laboratory services.
“We are excited to be involved in strengthening the laboratory system in Malawi to adequately serve the citizens of Malawi,” said Professor Alash’le Abimiku, the principal investigator for the AMPLIFY grant. “Our success in Malawi is a testimony of the incredible team we have on ground, of the true partnership and collaboration we have enjoyed with the Malawian government, CDC, and other implementing partners. Now we have to make sure that these accredited laboratories continue to operate at international standards in order to maintain their accreditation.”
Ciheb Malawi began working on laboratory accreditation in October 2019 and applied for accreditation for the four laboratories in April 2020. After an audit from SADCAS in October 2020, AMPLIFY supported the laboratories to address the nonconformities identified by SADCAS before the laboratories were awarded a two-year accreditation term in February 2021. AMPLIFY is currently supporting the rest of the seven HIV molecular laboratories and one tuberculosis laboratory to get accredited.
The first four accredited labs include:
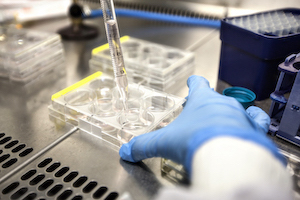
- National HIV Reference Laboratory, Lilongwe, Central Region
- Partners in Hope Laboratory, Lilongwe, Central Region
- Blantyre DREAM Laboratory, Blantyre, Southern Region
- Balaka DREAM Laboratory, Balaka, Southern Region
“This is a very big achievement,” said Dr. Visopo Harawa, Malawi deputy country director for Ciheb. “In Africa, there are very few laboratories that are accredited, and it takes a lot for a laboratory to reach the level of ISO accreditation, so it’s a very big achievement.”
 Meeting International Standards
Meeting International Standards
SADCAS, the regional accrediting body for 13 Southern African nations, assessed the four laboratories according to the ISO 15189 benchmarks. These standards cover personnel, equipment, documentation, external quality assurance, internal audits, customer care, safety, among other topics.
These four laboratories are primarily providing molecular diagnostic services for the PEPFAR-supported HIV program (viral load and early infant diagnosis testing), and more recently diagnosing SARS-CoV-2 from COVID-19 samples as well.
“Having your laboratory accredited is a great achievement and shows that your laboratory results are of good quality, the staff performing the testing are competent and certified, and the overall operations in the laboratory meet international standards,” said Sophia Osawe, a technical advisor from the Institute of Human Virology, Nigeria, who helped support the Ciheb Malawi team. “Additionally, this boosts the confidence of the laboratory and ensures that laboratory results are accurate, reliable, and timely, which are all crucial points for strengthening laboratory systems.”
A Quick Timeline
It didn’t take long for Ciheb Malawi to hit some key deliverables in the AMPLIFY project. The project originally planned for 10 labs to be accredited over a five-year period, at a rate of two per year, but the team has already surpassed expectations by accrediting four during its first year. This is a result of great partnership with the laboratories and the MOH ensuring efficient teamwork, resource investment, and high-quality technical support and mentorship.
Although the COVID-19 pandemic was a challenge in providing mentorship to the laboratories, the use of weekly virtual mentoring through Zoom proved effective in preparing the laboratories for the SADCAS assessment and working with them to address the nonconformities.
 Next: More Lab Accreditation
Next: More Lab Accreditation
Ciheb Malawi will conduct an internal audit in May to determine which of the remaining eight labs are closest to applying for accreditation. It hopes to get two more laboratories accredited by the end of 2021.
The remaining eight labs are spread across Malawi’s three regions:
- Northern Region: Rumphi District Lab, Mzuzu Regional Lab
- Central Region: Kamuzu Central Hospital Lab, National Tuberculosis Reference Lab
- Southern Region: Queen Elizabeth Central Hospital, Zomba Central Hospital, Thyolo District Lab, Nsanje District Lab
The experience from accrediting the first four labs will provide valuable knowledge and local resource for the remaining eight. The laboratories now have greater familiarity with the SADCAS accreditation process and understand what auditors are looking for and what problems areas to work on.