Cohort Event Monitoring
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has led to the development of vaccines recommended by the World Health Organization (WHO) for mitigating its impact. Vaccines approved for use in national immunization programs are considered safe and effective based on randomized controlled clinical trials. However, like all vaccines, adverse events may occur following COVID-19 vaccination, and therefore, vaccine safety surveillance is crucial to monitor vaccine safety in the general population and mobilize public trust through informed processes. In Nigeria, COVID-19 vaccination started in March 2021 with AstraZeneca, and the country has received other COVID-19 vaccines from other manufacturers/developers. Currently, adult individuals of 18 years and above are being primarily vaccinated at designated sentinel sites following the WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) recommendations.
 The WHO introduced the cohort event monitoring (CEM) program, which has been embraced by many developing countries, to bridge the safety data gap that exists between Phase Three clinical trials and routine passive surveillance. The CEM program actively tracks adverse events in individuals who have been exposed to new drugs and/or vaccines, providing a quick and easy way to collect data. This data offers reassurance about safety for regulators, EPI programs, and the public. The CEM evaluation was conducted in two phases and covered six states plus one additional area. Phase One took place in the FCT, Benin, Kaduna, Lagos, Enugu, and Bauchi, while Phase Two was conducted in the FCT, Benin, Kano, Lagos, Enugu, and Bauchi. The follow-up for CEM phase one lasted for 12 weeks, while Phase Two lasted for a year.
The WHO introduced the cohort event monitoring (CEM) program, which has been embraced by many developing countries, to bridge the safety data gap that exists between Phase Three clinical trials and routine passive surveillance. The CEM program actively tracks adverse events in individuals who have been exposed to new drugs and/or vaccines, providing a quick and easy way to collect data. This data offers reassurance about safety for regulators, EPI programs, and the public. The CEM evaluation was conducted in two phases and covered six states plus one additional area. Phase One took place in the FCT, Benin, Kaduna, Lagos, Enugu, and Bauchi, while Phase Two was conducted in the FCT, Benin, Kano, Lagos, Enugu, and Bauchi. The follow-up for CEM phase one lasted for 12 weeks, while Phase Two lasted for a year.
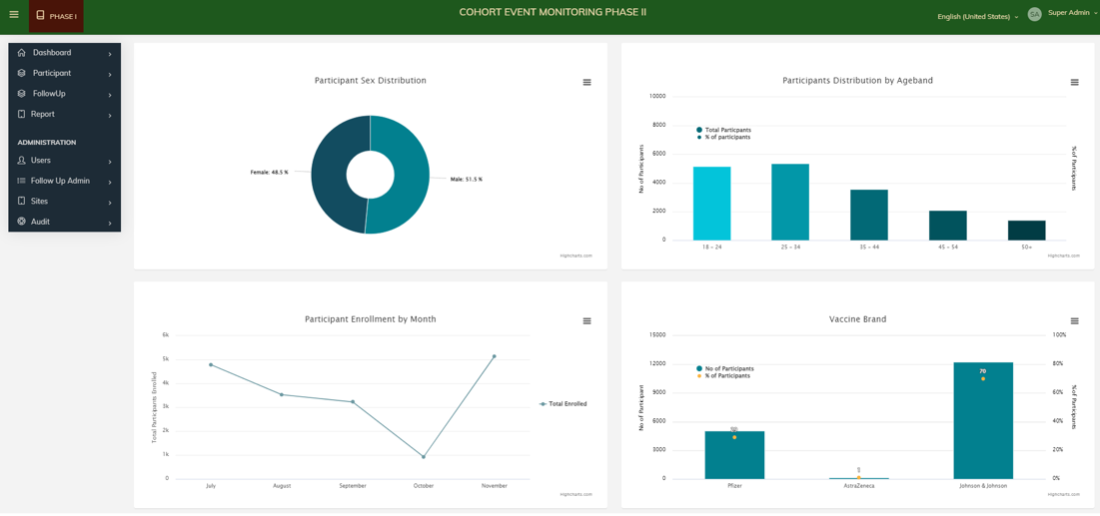 The 201 team has developed a CEM study application package that includes a CSPro-driven mobile application for data collection at vaccination facilities, a web application for follow-up clinicians to organize participants' schedules, an interfacing/integration layer for pushing data to Nigeria's surveillance system (Vigihub), and an analytics and visualization interface. This interface allows stakeholders to track the real-time progress of data collection and follow-up, as well as view summary charts that characterize adverse events following immunization (AEFI) among individuals who have received COVID-19 vaccines, including MAEs, SAEs, and AESIs. The team's survey and data collection capabilities are continually improving, making their CEM study application package more effective.
The 201 team has developed a CEM study application package that includes a CSPro-driven mobile application for data collection at vaccination facilities, a web application for follow-up clinicians to organize participants' schedules, an interfacing/integration layer for pushing data to Nigeria's surveillance system (Vigihub), and an analytics and visualization interface. This interface allows stakeholders to track the real-time progress of data collection and follow-up, as well as view summary charts that characterize adverse events following immunization (AEFI) among individuals who have received COVID-19 vaccines, including MAEs, SAEs, and AESIs. The team's survey and data collection capabilities are continually improving, making their CEM study application package more effective.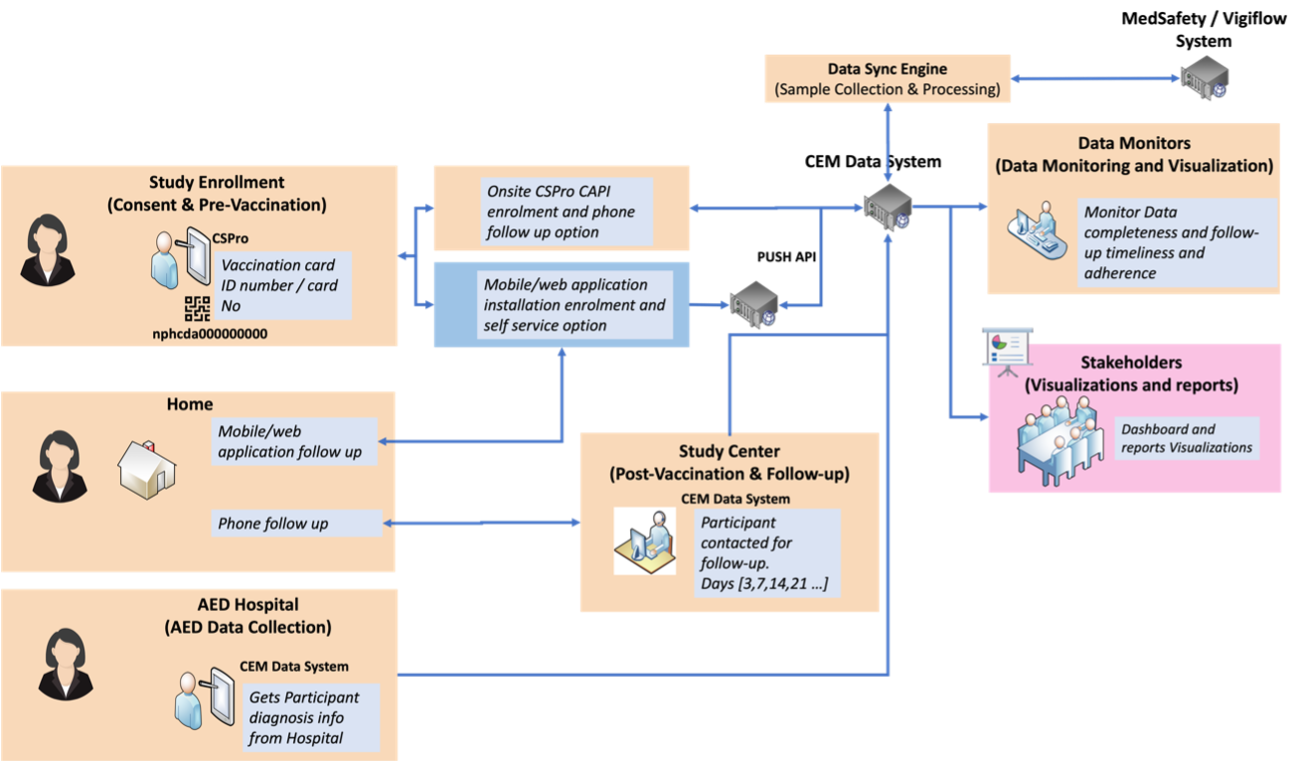
Both Phase One and Phase Two of the data collection process proceeded smoothly and on schedule thanks to the survey package developed by the 201 team. Stakeholders such as GON, CDC, and NAFDAC were able to access the analytics and visualization interface on the application in real-time, providing them with up-to-date information. The data collected was also sent to the Vigihub.
The CEM Data system was highly effective in both Phase One and Phase Two, enrolling over 12,000 and 17,000 participants, respectively. A substantial amount of follow-up data was collected, with 184,755 follow-up contacts in Phase One and 192,203 in Phase Two. For AEFI, Vigihub received data from 5,276 participants from both phases, indicating the remarkable efficiency of the system. Data collection is ongoing for the remaining participants, further emphasizing the effectiveness of the CEM Data system.